New drugs for Traumatic Brain Injury excite: Key takeaway from Assessment Analysis
HTF Market Intelligence released a new research report of 399 pages on title ‘Traumatic Brain Injury – Pipeline Review, H2 2019’ with detailed analysis, forecast and strategies. The study covers key regions and important players such as Acelerox LLC, ALSP Inc, AlzeCure Pharma AB etc.
Request a sample report @ https://www.htfmarketreport.com/sample-report/2360977-traumatic-brain-injury-pipeline-review
Summary
Global Markets Direct’s latest Pharmaceutical and Healthcare disease pipeline guide Traumatic Brain Injury – Pipeline Review, H2 2019, provides an overview of the Traumatic Brain Injury (Central Nervous System) pipeline landscape.
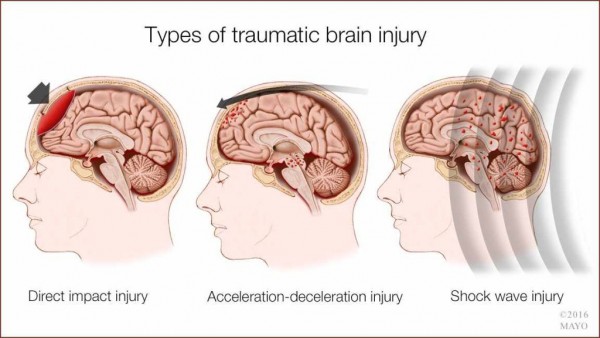
Traumatic brain injury happens when an external mechanical force causes brain damage. Traumatic brain injury usually results from a violent blow or jolt to the head or body. Symptoms include headache, dizziness, nausea or vomiting, mood changes, fatigue or drowsiness, difficulty sleeping and memory or concentration problems. Risk factor includes age. Certain types of traumatic brain injury increase the risk of developing Alzheimer’s disease and coordination problems.
Companies Mentioned in the Report
Acelerox LLC, ALSP Inc, AlzeCure Pharma AB, AMAG Pharmaceuticals Inc, Amarantus Bioscience Holdings Inc, Anagin Inc, Anida Pharma Inc, Annovis Bio Inc, AntiRadical Therapeutics LLC, Anvyl LLC, Aptinyx Inc, Astrocyte Pharmaceuticals Inc, Athersys Inc, Avanir Pharmaceuticals Inc, Bessor Pharma LLC, Beyond Barriers Therapeutics Inc, Biogen Inc, BioIncept LLC, Bioquark Inc, Brain-Gen LLC, CalciMedica Inc, CavoGene LifeSciences, CellCure, Cellvation Inc, CereSpir Inc, Chimerix Inc, Cognosci Inc, Complement Pharma BV, Deha Pharmaceutical LLC, Eisai Co Ltd, Epigen Biosciences Inc, Fortuna Fix Inc, GABA Therapeutics Inc, GNT Pharma Co Ltd, Hemarina SA, Hibernaid Inc, Hillhurst Biopharmaceuticals Inc, Hope Biosciences LLC, Hoverink Biotechnologies Inc, ImmunoChem Therapeutics LLC, Impression Healthcare Ltd, Inflammatory Response Research Inc, International Stem Cell Corp, Ischemix Inc, JT Pharmaceuticals Inc, Kalytera Therapeutics Inc, KannaLife Sciences Inc, LA Cell Inc, Levolta Pharmaceuticals Inc, Mapreg SAS, Mercaptor Discoveries Inc, MetVital Inc, NeuExcell Therapeutics Inc, NeurAegis Inc, Neuren Pharmaceuticals Ltd, NeurExo Sciences LLC, Neurodon LLC, Neuronasal LLC, NeuroNascent Inc, Neurotrope Bioscience Inc, NeuroVive Pharmaceutical AB, New World Laboratories Inc, NoNO Inc, NuvOx Pharma LLC, Nyrada Inc, Orpheris Inc, Oxeia Biopharmaceuticals Inc, Peptron Inc, PharmatrophiX Inc, Praetego Inc, Prevacus Inc, ProNeurogen Inc, ProThera Biologics Inc, Qrons Inc, Radikal Therapeutics Inc, RegeneRx Biopharmaceuticals Inc, Resolys Bio Inc, RHNanopharmacuticals LLC, Rubicon Biotechnology Inc, SanBio Inc, Seneca Biopharma Inc, Shinkei Therapeutics LLC, Silver Creek Pharmaceuticals Inc, Spinogenix Inc, STATegics Inc, Stemedica Cell Technologies Inc, Swedish Orphan Biovitrum AB, Tellus Therapeutics Inc, Tetra Therapeutics, Thera Neuropharma Inc, Therapeutic Solutions International Inc, Therapix Biosciences Ltd, Theratome Bio Inc, TikoMed AB, Tiziana Life Sciences Plc, Tria Bioscience Corp, vasopharm GmbH, VG Life Sciences Inc, Virogenomics BioDevelopment Inc, Xcelthera INC, Xonovo Inc, ZyVersa Therapeutics Inc
View Detailed Table of Content @ https://www.htfmarketreport.com/reports/2360977-traumatic-brain-injury-pipeline-review
Report Highlights
Global Markets Direct’s Pharmaceutical and Healthcare latest pipeline guide Traumatic Brain Injury – Pipeline Review, H2 2019, provides comprehensive information on the therapeutics under development for Traumatic Brain Injury (Central Nervous System), complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA) and molecule type. The guide covers the descriptive pharmacological action of the therapeutics, its complete research and development history and latest news and press releases.
The Traumatic Brain Injury (Central Nervous System) pipeline guide also reviews of key players involved in therapeutic development for Traumatic Brain Injury and features dormant and discontinued projects. The guide covers therapeutics under Development by Companies /Universities /Institutes, the molecules developed by Companies in Phase III, Phase II, Phase I, IND/CTA Filed, Preclinical and Discovery stages are 1, 8, 8, 1, 89 and 15 respectively. Similarly, the Universities portfolio in Preclinical and Discovery stages comprises 7 and 2 molecules, respectively.
Traumatic Brain Injury (Central Nervous System) pipeline guide helps in identifying and tracking emerging players in the market and their portfolios, enhances decision making capabilities and helps to create effective counter strategies to gain competitive advantage. The guide is built using data and information sourced from Global Markets Direct’s proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations and featured press releases from company/university sites and industry-specific third party sources. Additionally, various dynamic tracking processes ensure that the most recent developments are captured on a real time basis.
Scope
– The pipeline guide provides a snapshot of the global therapeutic landscape of Traumatic Brain Injury (Central Nervous System).
– The pipeline guide reviews pipeline therapeutics for Traumatic Brain Injury (Central Nervous System) by companies and universities/research institutes based on information derived from company and industry-specific sources.
– The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration till discovery and undisclosed stages.
– The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
– The pipeline guide reviews key companies involved in Traumatic Brain Injury (Central Nervous System) therapeutics and enlists all their major and minor projects.
– The pipeline guide evaluates Traumatic Brain Injury (Central Nervous System) therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA) and molecule type.
– The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
– The pipeline guide reviews latest news related to pipeline therapeutics for Traumatic Brain Injury (Central Nervous System)
Reasons to buy
– Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
– Recognize emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage.
– Find and recognize significant and varied types of therapeutics under development for Traumatic Brain Injury (Central Nervous System).
– Classify potential new clients or partners in the target demographic.
– Develop tactical initiatives by understanding the focus areas of leading companies.
– Plan mergers and acquisitions meritoriously by identifying key players and it’s most promising pipeline therapeutics.
– Formulate corrective measures for pipeline projects by understanding Traumatic Brain Injury (Central Nervous System) pipeline depth and focus of Indication therapeutics.
– Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
– Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from pipeline.
Get Customization in the Report, Enquire Now @ https://www.htfmarketreport.com/enquiry-before-buy/2360977-traumatic-brain-injury-pipeline-review
Table of Contents
Introduction
Traumatic Brain Injury – Overview
Traumatic Brain Injury – Therapeutics Development
Traumatic Brain Injury – Therapeutics Assessment
Traumatic Brain Injury – Companies Involved in Therapeutics Development
Traumatic Brain Injury – Drug Profiles
Traumatic Brain Injury – Dormant Projects
Traumatic Brain Injury – Discontinued Products
Traumatic Brain Injury – Product Development Milestones
AppendixList of Tables
….Continued
Buy this report @ https://www.htfmarketreport.com/buy-now?format=1&report=2360977
Thanks for reading this article, you can also get individual chapter wise section or region wise report version like North America, Europe or Asia.
Media Contact
Company Name: HTF Market Intelligence Consulting Private Limited
Contact Person: Craig Francis
Email: Send Email
Phone: 2063171218
Address:Unit No. 429, Parsonage Road
City: Edison
State: New Jersey
Country: United States
Website: https://www.htfmarketreport.com/reports/2360977-traumatic-brain-injury-pipeline-review



